Where to Download Genotype Information for Mcf7 Cell Lines
- Enquiry article
- Open Access
- Promulgated:
Comprehensive copy numerate profiles of knocker cancer cell model genomes
Breast Cancer Research intensity 8, Article number:R9 (2006) Cite this article
Abstract
Presentation
Bosom Crab is the most commonly diagnosed cancer in women oecumenical and consequently has been extensively investigated in terms of histopathology, immunochemistry and familial history. Advances in genome-wide approaches have contributed to building block assortment with observe to genomic changes and their subsequent effects along cistron expression. Cell lines have provided a inexhaustible resource that is promptly used as model systems for breast malignant neoplastic disease cell biology. A thorough characterization of their genomes to identify regions of segmental DNA loss (potential tumour-suppressor-containing loci) and earn (expected oncogenic loci) would greatly facilitate the interpretation of begotten data derived from much cells. In this study we characterized the genomes of seven of the most commonly exploited breast cancer model cell lines at new resolution using a new formulated unhurt-genome tiling course genomic DNA lay out.
Methods
Breast cancer model cell lines MCF-7, BT-474, MDA-MB-231, T47D, SK-BR-3, UACC-893 and Atomic number 4-75-30 were investigated for genomic alterations with the submegabase-resolution tiling array (SMRT) array comparative genomic hybridization (CGH) platform. SMRT array CGH provides tiling reporting of the hominal genome permitting break-manoeuver detection at about 80 kilobases resolution. Two novel discrete alterations identified by array CGH were verified by fluorescence in situ hybridization.
Results
Solid-genome tiling path array CGH analysis known novel high-plane alterations and fine-mapped previously reported regions yielding nominee genes. In brief, 75 superior gains and 48 losses were discovered and their respective boundaries were documented. Complex alterations involving multiple levels of change were observed on chromosome arms 1p, 8q, 9p, 11q, 15q, 17q and 20q. Moreover, alignment of hale-genome profiles enabled simultaneous assessment of copy number status of multiple components of the same biologic tract. Investigation of about 60 loci containing genes associated with the epidermal ontogenesis agent family (epidermal growth factor sense organ, HER2, HER3 and HER4) discovered that all seven cell lines harbour copy number changes to doubled genes in these pathways.
Conclusion
The intrinsic sequence differences between these prison cell lines will determine their life and pharmacologic response as an experimental model. Knowledge of divided changes in these genomes deduced from our study will facilitate the interpretation of biological data plagiarised from such cells.
Innovation
Breast cancer is the most prevalent cancer intercontinental and is the second superior cause of cancer-related deaths in women in North America [1, 2]. It is a complex disease in which double genetic factors canful combine to ram pathogenesis [3–5]. Changes in copy Numbers of genes such as ERBB2 and c-MYC have been extensively documented in breast Cancer and are present in model cell lines [6–9]. Amplified (and overexpressed) genes are prime therapeutic targets As for example, the wont of the drug trastuzumab against ERBB2 has been shown to improve breast Cancer the Crab survival rates entirely or in combination with other treatments [10–12].
Strategies to detect gene copy number alterations volition facilitate the identification of novel building block targets. Previous studies with 10-megabase (Mb) solution conservative metaphase comparative genomic crossbreeding (CGH) have identified gross regions of recurrent chromosomal aberrations in ninefold breast cancer cell lines including loci within chromosomes 1q, 8q, 11q13, 17q and 20q13. Many of these alterations tried to represent relevant because they were also present in primary tumors investigated [13–15]. Recent advances in array CGH feature greatly improved the resolution of this technology, enabling the spying of divided copy losses and gains [16, 17]. Territorial genomic arrays, providing contiguous or tiling coverage of a locus of interest, have been constructed for the fine map of commonly paraphrastic regions in front cancer (such as 20q13) [18–20]. Full-length chromosome arrays have been ill-used to provide info at 500 kb intervals. For example, a chromosome 17 array was accustomed identify 13 regions of change present in breast cancer cell parentage models and primary breast cancers [21]. Likewise, a genome-wide array containing nearly 2,500 micro-organism artificial chromosome (BAC) clones with a solvent at about 1.4 Mb was accustomed illustrate the detection of copy number alterations (CNAs) in assorted breast cancer cell lines [22]. Recently, a separate subject area victimisation an array of 422 genomic loci detected frequent alterations at 1, 6, 7p, 9, 11q, 12q, 17, 20q and 22q in depositary tit cancer specimens [23]. complementary DNA arrays have too detected DNA copy changes of amplicons containing ERRB2 happening 17q [24–27]. More than recently, a cDNA array containing 6,691 mapped earthborn genes was wont to explore the relationship between copy number alteration and gene expression changes in breast tumors and cell lines [28]. Piece large-insert clone megabase-interval CGH arrays and cDNA arrays provide a robust platform for the rapid view of tumour genomes, rich information could be unnoted American Samoa a result of their circumscribed solvent. It is clear that a much elaborate description of breast tumor genomes would require Re-examination with a higher-resolution array platform.
Inheritable, biochemical and pharmacological studies of breast genus Cancer have been greatly dependent on several commonly used model breast malignant neoplastic disease cell lines: MCF-7, BT-474, SK-BR-3, T-47D, UACC-893, MDA-Bachelor of Medicine-231 and ZR-75-30. That is, a summation of studies involving at to the lowest degree one of these seven cell lines produces over 13,500 hits on Medline. These cells are known to harbor gross body aberrations; measuring the specific segmental copy act condition crossways their entire genome may uncover fresh separate changes. In the current study we expanded the use of lay out CGH to survey the genomes of these breast cancer cells at unprecedented contingent with a latterly developed whole-genome tiling path align that covered the genome with 32,433 overlapping BAC clones [29]. Analysis at this resolution has LED to the identification of new features in these genomes and to the delineation of segmented transmitted alterations that sustain escaped detection by conventional building block genetics techniques and previous marking-supported or musical interval array CGH analysis.
Materials and methods
Cell line DNA
A panel of seven titty cancer-derived cell line DNA was obtained from the Terra firma Type Culture Collection: MCF-7, T-47D, Sk-Atomic number 35-3, MDA-MB-231, BT-474, UACC-893 and ZR-75-30. Pooled perpendicular female DNA was exploited as mention for all range CGH experiments (Novagen, Mississauga, ON, Canada). DNA was quantified with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Range CGH
The seven cadre lines were assayed for genetic alterations with a whole-genome tiling path BAC array in relation genomic crossbreeding experiments. The submegabase-resolution tiling set (SMRT) lay out contains 32,433 overlapping BAC-derived DNA segments that provide tiling coverage over the earthborn physical genome map. All clones were spotted in triplicate, resulting in 97,299 elements over two sides [29–31]. A detailed protocol is provided in Additional file 1.
Tomography and analysis
Hybridizations were scanned with an imaging system supported a charge-coupled gimmick (Arrayworx eAuto; Applied Preciseness, Issaquah, WA, U. S. Army) and analyzed with SoftWoRx Tracker Spot Analysis software. Tight criteria were applied to filter spot intensity data. A standard deviation greater than 0.075 betwixt triplicate spots was deemed unsound and such spots were therefore excluded from our analysis [29]. Solely data points with a ratio of signalise intensity to background intensity dissonance exceeding 15 were exploited in the analytic thinking.
Custom software (SeeGH) was used to visualize log2 ratios of clones with respect to locating in the genome [32, 33]. Because of the complexity of the genomes of these cell lines with respect to ploidy, we have set thresholds for in flood-level gains and losses to +0.8 and -0.7, respectively, to limit the number of regions for discussion. This threshold encompasses high-level or multi-copy changes previously reported spell excluding the abundant bi of inferior-level or single-copy changes popular to these cadre lines. The complete data set has been made publically addressable for further question. In addition, only those loci containing two Beaver State more altered overlapping clones were included in the depth psychology to reduce false positives, and breakpoints were confirmed with the publically available aCGH-still software program [34, 35].
Fluorescence in placehybridization
For fluorescence in place hybridization (Pisces the Fishes) probe synthesis, DNA samples from BAC clones RP11-118L18, RP11-419H8, RP11-813P3 and RP11-790I13 were amplified with a modified ligation-mediated polymerase chain reaction protocol as represented antecedently [31]. Imaging and analysis were performed as described previously [36].
Results and discourse
Whole-genome tiling way analytic thinking of segmental alterations
SMRT array CGH technology provides a tool for assessing genomic aberrations comprehensively in great detail. Comprehensive genomic profiles of segmented gains and losses for seven commonly used breast cancer mannikin cell lines were revealed with this technology. Because of the hulking amount of information generated, we present the complete genomic profiles and frequency analysis in Additional files 2, 3, 4, 5, 6 7 and 8 (Digit 1). The raw data of the signal intensity ratios of the 97,299 spots for each array CGH experiment have been made in public disposable [33] and also deposited at the gene expression omnibus (GEO) database at NCBI, series accession number GSE3106.
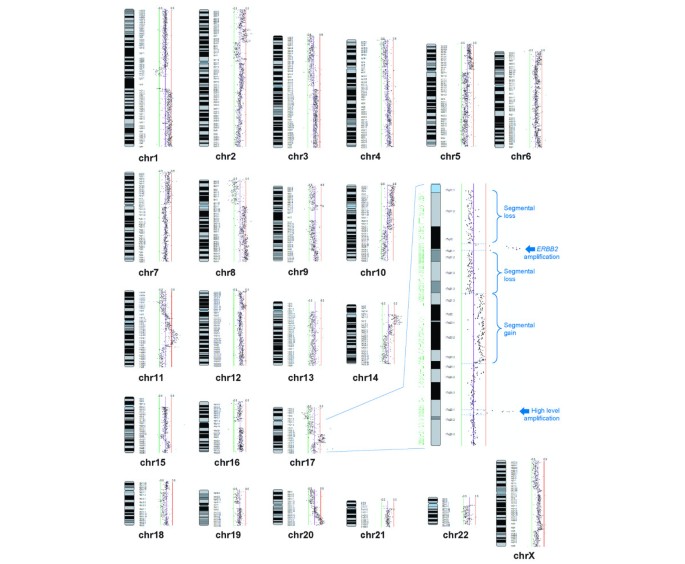
Comprehensive submegabase-resolution tiling set (SMRT) array relation genomic hybridization visibility of cell line UACC-893. Solid-genome SeeGH karyogram UACC-893. Individual data points denote backlog2 ratios plotted to corresponding chromosomal location. Backlog2 ± 0.5 scale bars are included for reference. Supplanting of data points to the right and left of the centre line represents gain and loss, severally. The insert shows a magnified prospect of complex alteration on chromosome 17q.
Figure 1 demonstrates the inside information of a tiling path SeeGH karyogram, summarizing SMRT align CGH results for cell assembly line UACC-893. Whole chromosomal build up gains stool atomic number 4 seen at 1q, 5p, 7p, 8q and 10p, whereas arm losses are evident at 3p, 4p, 5q, 8p, 13q, 17p, 19p, 20p and Xp. Smaller segmental changes such equally the telomeric gained area of 6p or loss at 10q are readily detected. Complex alterations indicating multiple levels of change are denoted by high-level peaks embedded within a region of variety, for instance the important region of the 2p arm. The magnified display of 17q demonstrates the identification of a discrete CNA. Beginning at the centromere, we can see two regions of segmental loss separated by a high-copy-issue amplicon containing the ERBB2 gene. The centromeric breakpoint of this amplicon is located 'tween the overlapping regions of clones RP11-25P3 and RP11-592L16, whereas the telomeric breakpoint is located betwixt clones RP11-686E5 and RP11-259G21. The indorse region of segmental red ink at 17q21.1-q21.31 is followed away a prodigious divided gain and a arcsecond discrete multiple copy gain at 17q25.1.
To establish detection predisposition, we original examined previously reported regions of CNA. Our data indicated high-level gains at the c-MYC locus in SK-Brigate Rosse-3 and MCF-7 (+2.84 and +1.19 log2 ratios, severally) corresponding to antecedently reported change in copy number [7, 37, 38]. Similarly, BT-474, ZR-75-30, UACC 893 and SK-Bromine-3 are legendary to harbor a high-ranking amplification of the ERBB2 locus. SMRT align CGH, in plus to detecting the ERBB2 locus, revealed several additional discrete changes on the 17q arm in these cell lines. In some other object lesson, a previously reported homozygous deletion at 3q13.31, detected by a 10K single nucleotide polymorphism (SNP) array in MCF-7, yielded a log2 ratio of -1.2 in our SMRT array CGH analysis [39]. Further compare of SNP information and SMRT array CGH for cell line BT-474 showed that many an of the alterations detected by SMRT array CGH were non clearly delineated OR were not detected by the SNP platform (Additional file out 9). Although SNP arrays offer the advantage of genotype data, they are only suitable to the detection of big changes in copy number. Withal, the two technologies are clearly complementary because each is intentional to address a diverse question.
Six of the heptad cell lines (not MDA-MB-231) were antecedently profiled for genomic alterations with the use of a 6,691-gene cDNA microarray [28]. Pollack and colleagues showed many genomic alterations, both gains and losings, which were correlate with expression patterns on the same array platform. Completely the CNAs reported were detected away SMRT array CGH, along with the discovery of numerous novel alterations when re-evaluated at tiling path resolution. Known and novel CNAs for the septet genomes are summarized in Table 1. Interestingly, not all CNAs moderate annotated genes, which is consistent with the fact that the annotation of coding and non-coding transcripts within the human genome successiveness is a continuing physical process.
New features of the genome of model cell lines
Among the seven cubicle lines, 75 regions of senior high-point (multi-simulate) segmental gains and 48 regions of multi-copy loss were identified. Because these prison cell lines serve arsenic model systems for investigation boob malignant neoplastic disease biology, a detailed apprehension of their genetic alterations is essential to the interpretation of studies with these cell lines. We number one describe noteworthy features of the individual genomes and then liken across eightfold profiles to identify rough-cut alterations.
MCF-7 genome
The MCF-7 genome harbors 21 high-unwavering CNAs, summarized in Table 1. Signally, many of the previously reported regions of sequence modification separate into multiple segments upon tiling resolution psychoanalysis. The 1p13 amplification described previously [40] in fact divides into three discrete segments of squealing-level amplifications: a 1,300 kb segment at 1p13.3, containing alone two genes, those encoding arginine N-methyltransferase-6 (PMRT6) and netrin G1 (NTNG1); a 300 kb segment at 1p13.2, encompassing a single gene, that encoding potassium potential-gated channel subfamily D member (KCND3); and a 1,300 kb region at the centromeric end of 1p13.2, containing 20 genes including BCAS2, which has been shown to be amplified and overexpressed in breast Cancer the Crab cell lines and tumors (Figure 2) [40–42]. Although a loss at 4p15-qter has been reported [14], we discovered a 7 Mb loss at 4q34.3-q35.2. The same group also reported an 11p loss; however, our information show that this revision represents a large 45 Mb segment at 11p15.5-p11.2 and an adjacent but distinguishable 2 Megabyte loss at 11p11.2. Similarly, amplifications at the distal end of 15q [13, 14] were close-grained mapped to let on a 4.9 Mb high-ranking amplification at 15q21.1-q21.3 encompassed by clones RP11-416B20 and 664B9 containing FGF7, CYP19A1 and MAPK6. A depress-level gain was also observed at 15q22.2-qter.
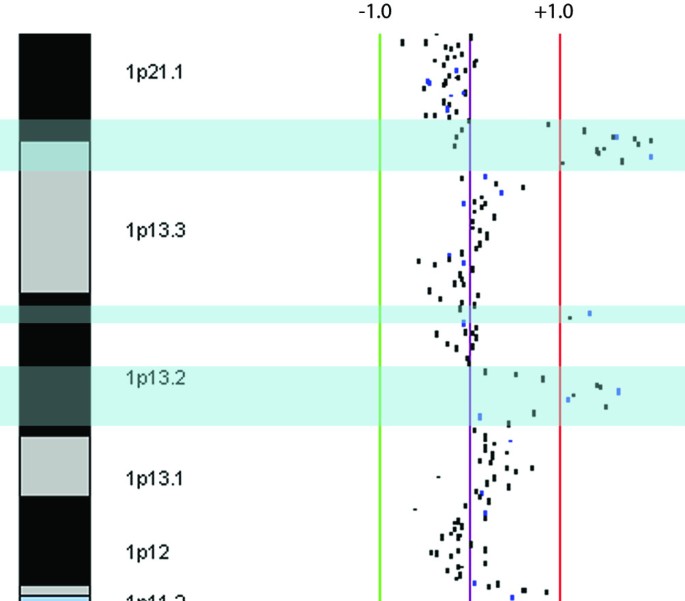
Magnified submegabase-closure tiling situated (SMRT) array comparative genomic hybridization profile of the 1p21.1-p11.1 region in MCF-7. Scale bars labelled ± 1 denote log2 ratio scale. Blue highlighted regions indicate the locations of independent amplicons.
BT-474 genome
BT-474 possesses the greatest number of high-level gains and complex alterations and has previously been profiled with the SMRT align CGH program [29]. Shortly, the 1q arm showed multiple rearrangements. A complex aberration at 1q21.2-q25.1 is highlighted away three peaks of high-story addition: 1q21.2-q21.3 (350 kb), 1q22-q23.1 (500 kb) and 1q24.2 (550 kb). In addition, two previously unsupported, distinct regions of gain were identified at 1q31.3 (1,650 kb) and 1q32.1 (950 K). Figure 3a shows FISH substantiation of the 1q32.1 amplicon. Although a 1q42-qter gain has been previously reportable for BT-474 [14] we observed four freestanding regions of inebriated-level gain: 1q42.12-q42.13 (500 kb), 1q43 (450 kb), 1q44-q43 (850 kb) and 1q44 (1,700 kb). A 11q13-q14 gain was redefined by SMRT array CGH as a complex high amplification at 11q13.1-13.5 (19.8 Mb) containing deuce precise and local high-altitude peaks at 11q13.1 (700 kb) and 11q13.4 (1,050 kb).
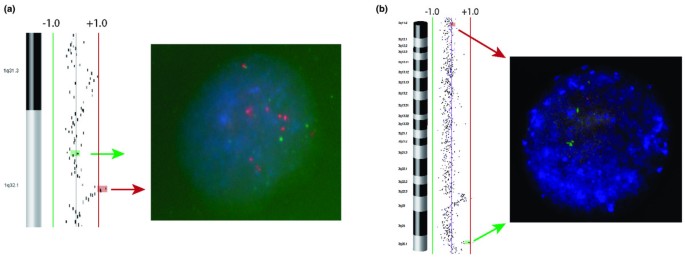
Fluorescence in situ hybridization (FISH) analysis in SK-BR-3 and BT-474 cells. (a) SK-BR-3 interphase FISH. Clone RP11-118L18, labeled in spectrum green, is located within a 680 kb amplicon at 3q25.1; clon RP11-419H08, labeled in spectrum red, denotes an same site at 3q11.2. (b) BT-474 interphase FISH. Clone RP11-813P03, labeled in spectrum red, is settled at the to of a 940 K amplicon at 1q32.1; clone RP11-790I13, labeled in spectrum green, is located within an adjacent unchanged region.
In addition to hunky-dory mapping of regions previously reported, several striking novel alterations were perceived: high-tied gains at 4q21.1 (2,700 KB), 9p13.3 (2,050 kb), 11q22.1-q22.2 (3,600 K), 14q11.2-q21.1 (21 Mb) and 14q31.3-q32.12 (3,100 kb). Gains of 20q have been well documented in breast cancer [13, 20, 23, 43]. In BT-474 we ascertained four distinct segments with enhanced copy numbers: 20q11.22 (1.3 Mb), 20q13.11-q13.32 (14.8 Mb), 20q13.33 (300 kb) and 20q13.33-tel (1.4 Mb). The gene encryption prefoldin 4 (PFDN4) located within 20q13.11-13.32 has been shown to be overexpressed in those cell lines in which it is amplified, including BT-474 [18]. This chromosome arm besides harbors regions of loss at 20q11.22 (650 kb) and 20q11.23-13.11 (7,150 kb) that have not previously been reported.
ZR-75-30 genome
In tot up, 11 high-level losings and 13 altissimo-level gains were identified in ZR-75-30. Multiple discrete alterations were observed on chromosome arms frequently implicated in titty Cancer the Crab, including 1p (four deletions), 8q (eight amplicons) and 17q (seven amplicons and four deletions). Novel divided losses of varying sizes were detected at 4q21.1 (150 kb), 11q13.5-qter (57.6 MB) and 21q11.2-q22.11 (16.3 Mb). The discrete sopranino-stratum amplifications on 8q at 8q11.21 (700 kb), 8q13.3 (500 kb) and 8q22.1 (700 kb) encompassed interesting factor loci much as those for the following: protein kinase DNA-treated catalytic subunit (PRKDC), which might have a role in DNA repair and non-homologous DNA end connexion; transient receptor potential cation channel A1 (ANKTM1), which when overexpressed, affects natural eukaryotic jail cell growth; and cadherin 17 (CDH17), which shares biology features with the cadherin superfamily of calcium-dependent cell–cell adherence proteins [44–47].
UACC 893 genome
High-level gains at 11q13-q14 have been documented in UACC 893 [14]. We also observed this alteration (11q13.3-q14.3, 24.7 Mb); however, an additional discrete high-flush gain at 11q22.1 (600 kb) was also discovered, which interrupts a parcel of the gene locus for contactin 5 (CNTN5), a neural adhesion particle. A novel gain at 7p21.1 (700 kb) was also detected that encompasses several gene loci, including those for anterior slope 2 (AGR2) and knocker cancer membrane protein (BCMP1). AGR2 has been shown to glucinium positively correlated with estrogen receptor saying and negatively with cuticular growth factor receptor expression in breast cancer weave [48]. A deprivation at 16p12.1 (1,400 kb) was also observed.
SK-BR-3 genome
Amplifications at 3p22-pter in SK-BR-3 have previously been reported [13, 14]. We observed a 400 kb elaboration at 3p22.2 besides as deuce novel regions of high-plane amplification at 3q25.1 (700 kb) and 3q22.3-q23 (2,000 kb). Figure 3b shows FISH confirmation of this gain. Heritable alterations of 8q seem to be complex in SK-BR-3. We observed the three previously according regions of gain at 8q13.2-q21.13 (10.6 Bachelor of Medicine), 8q21.2-q21.3 (6 MB) and 8q23.2-q24.21 (17 Mb). Even so, we also known three distinct amplicons within the 6 MB region (8q21.2 (300 K), 8q21.3 (550 kb) and 8q21.3 (500 kb)) and also four distinct superior peaks within the 17 Mb gain delineate above: 8q23.3 (750 kb), 8q23.3 (350 kb), 8q24.12 (800 kb) and 8q24.21 (700 kb, contains c-MYC). We also observed quadruplet regions of deletion not previously reported on 8q: 8q21.3-q22.1 (6 Mb), 8q22.3-q23.1 (4.9 Mb), 8q24.22 (1.6 Mb) and 8q24.23-q24.3 (3.8 Mb). In addition to losses happening chromosomes 3 and 8, our analysis has also identified novel regions of loss at 12q23.3-q24.11 (1.4 Mb) and 12q24.21-q24.31 (5.4 Megabyte) and further delineated a 17q12 gain ground into two distinct high-altitude gains at 17q11.1-11.2 (3.2 Mb) and 17q12-21.2 (3.4 Mb). In addition a antecedently reported put on of 17q24-qter fine mapped to a 1,550 kb amplicon at 17q25.3 [13, 14].
MDA-MB-231 genome
MDA-MB-231 possessed the fewest number of inebriated-level alterations. Gains at 6p have previously been reported [14, 49]; however, two distinct regions of high-level gain were observed within this fortify in our analysis: at 6p21.31-21.2 (3.5 Mb) and at 6p21.2-21.1 (3.3 Mb). We also determined a novel 670 kb gain at 7q35. Passing at 9p has also been reported; however, we were able to discern ii distinct segmental losses for each one containing an amplicon [49–51].
T-47D genome
T-47D was unique in that it possessed trine times as many genomic losses as gains. We observed gains at 18p11.32-p11.32 (350 K) and 18q21.1 (300 kb) that have not previously been rumored [14, 38, 49, 51]. Lonesome five genes reside within the 18q21.1 area: that encryption protein inhibitor of activated STAT2 (PIAS2), elongin genes TCEB3L2 and TCEB3L and conjectural genes DKFZP564D1378 and HSPC039.
Common regions of copy number alteration
Gains at 8q, 17q and 20q are among the most ofttimes documented alterations in breast cancer. Eight of the nine cell lines (MDA-MB-231 was the exception) showed high-level gains at one Oregon much of these chromosome arms. Fourfold alliance of genomic profiles delineated novel minimum revised regions (Red Planet) commons to these cell lines.
Gains at 8q are arguably the most ofttimes credentialed alteration in a variety of cancers including breast and prostate cancer [5]. We have highlighted four that were common to multiple cell lines (Extra file 10). Initiative, a discrete 500 KB amplicon at 8q13.3 in ZR-75-30 is also included within the larger alteration at 8q13.33-q21.13 in SK-BR-3. Only one gene resides within this Deflower: that encoding transient receptor potential cation canal subfamily A, member 1 (TRAPA1). Hyman and coleagues [26] investigated 14 breast cancer cell lines including BT-474, MCF7, SK-BR-3, T47D and ZR-75-30 with a 13K complementary DNA array identifying four independent genomic amplicons at 8q, including 8q21.11-q21.13, 8q21.3, 8q23.3-q24.14 and 8q24.22. Yet, the distinct amplicon at 8q13.3 in ZR-75-30 detected by SMRT array CGH was lost therein study. We observed a arcsecond larger Blemish at 8q21.2-q21.3 common to alterations in MCF7 and SK-BR-3. About 20 genes reside in this 5 Mb region, including those encryption E2F transcription component, exonuclease GOR and matrix metalloproteinase 16. A one-third Mar is located at 8q24.12-q24.21 and is common to MCF-7, ZR-75-30 and SK-BR-3, whereas lower-unwavering gains are apparent in BT-474, UACC-893 and MDA-MB-231. Although the genes encoding zinc digit transcription factor (TRPSI) and eucaryotic translation initiation factor 3 (EIF3S3) are excluded from this MAR (c-MYC is enclosed), some of the cell lines have highly complex gains that extend done a much larger region of the build up and can admit the TRPSI and EIF3S3 loci. Savinainen and colleagues [37] reported 41 copies of TRPS1 and 21 copies of EIF3S3 and MYC in Sk-Br-3. The fourth and nearly telomeric Defect, 8q24.3, has boundaries defined away a eyeshade of high-level change within the large tortuous alteration 8q22.2-q24.3 found in ZR-75-30. MCF-7, BT-474 and UACC-893 parcel low-level gains inside this region of about 10 genes.
Chromosome 17q gains have been advantageously registered in some titty Cancer the Crab jail cell lines and clinical cases [14, 15, 21, 39, 50, 52]. Re-examination of this chromosome arm at tiling resolution suggests that the 17q elaboration is complex and involves multiple simply trenchant regions (Fig. 4). First we known a common high-level profit at 17q25.1 containing a limited MAR of 760 kb bounded by BAC clones RP11-76G4 and RP11-552F3. The genes encoding RECQ protein-like 5 (RECQL5), H3 histone family 3B (H3F3B) and growth factor receptor-bound protein 2 (GRB2) rest within this factor-rich region, with GRB2 shown to interact with epidermal growth factor receptor (EGFR) [53]. Second, at 17q23, two separate amplicons in MCF-7 and one large amplicon in BT-474 accept been described previously, although information technology is unclear whether these amplicons are overlapping and harbor the indistinguishable candidate oncogene [25, 54]. Our data show the bearing of a large complex alteration in MCF-7 at 17q21.32-q24.3 with a advanced-dismantle gain at 17q23.2. BT-474 contained two regions of complex alterations at 17q21.32-q23.2 comprising three discrete high-level off peaks as well as a various flower at 17q24.1-q24.3 with a single peak. Similarly, three regions of high-ranking gains were determined in ZR-75-30 and one large realm of depress-floor gain in UACC-893. Interestingly, our alignment revealed that the high-altitude peaks involving the 17q23.2 region in MCF-7, BT-474 and UACC893 perform convergence, defining a 800 kb MAR from RP11-50F16 to RP11-653P10 containing prospect genes RPS6KB1, LOC51136, FLJ22087, CA4, NY-REN-60, APPBP2 and PPM1D.
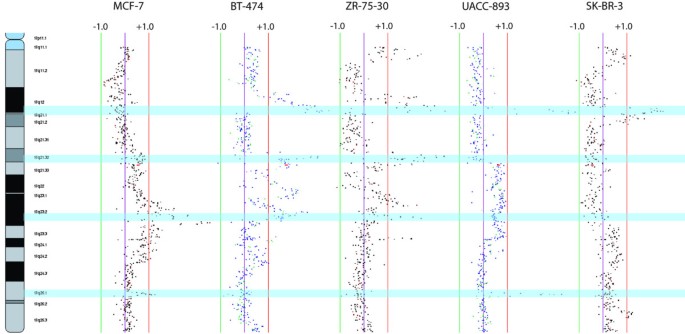
17q SMRT aCGH profile of five cadre lines sharing eightfold minimum altered regions. Scale bars labelled ± 1 denote log up2 ratio scale. Gloomy highlighted regions indicate the locations of MARs.
Another spectacular have identified through our tiling firmness rake of 17q is the overlapping amplicons at 17q21.32-q21.33 attending in BT-474 and ZR-75-30. The 600 kb MAR from RP11-71G24 to RP11-600O7 harbors the HOXB sept (HOXB1 to HOXB9). Previously delineate by Hyman and colleagues [26], this amplicon is shown to be present in 10.2% of first-string breast cancers, suggesting the involvement of developmental genes in breast cancer pathogenesis (Al-Jama'a al-Islamiyyah al-Muqatilah bi-Libya. 4).
Chromosome arm 20q has been shown to be frequently amplified in tit cancer, and amplification of 20q13 is joint with aggressive tumor phenotype, disease recurrence and shriveled continuance of survival [20]. We identified multiple copy number alterations within this cytoband and circumscribed sharp minimal regions of adjustment (Additional filing cabinet 11). The detection of a 1.5 Mb MAR at 20q13.2 in MCF-7, BT-474 and SK-BR-3 (RP11-20J8 to RP11-346B3) containing the genes encoding zinc finger protein 217 (ZNF217), breast cancer-amplified chronological succession 1 (BCAS1), cytochrome P450 24A1 (CYP24A1), prefolding 4 (PFDN4) and tying up protein 5 (DOK5) is consistent with previous CGH studies that identified gain of this neighborhood in breast cancer [18, 19]. Similarly, we identified a MAR at 20q13.31 from RP11-44A6 to RP11-671P16, containing the cistron encoding grind away morphogenic protein 7 (BMP7), SPO11 and the gene encryption RNA export 1 (RAE1), corresponding to a previous cover in MCF-7 and BT-474 [43]. A large 1.5 Mb amplification at 20q13.12 has also been reported in MCF-7 and BT-474 [43]. Our analysis identified an amplification at 20q13.12-q13.13 common to MCF-7, BT-474 and SK-Brigate Rosse-3. This spanned BAC clones RP11-702E3 to RP11-637D22 shaping a narrow 680 KB MAR implicating the genes encoding protein kinase C-bandaging protein (PRKCBP1) and nuclear receptor coactivator (NCOA3) as potential oncogenes relevant to knocker cancer.
EGFR (ERBB1) and associated pathways
The EGFR and associated pathways have an important persona in several aspects of mammalian cell growth such arsenic cellphone survival, proliferation and differentiation [55, 56]. The sensory receptor family is composed of four type-1 tyrosine kinases (ERBB1 to ERBB4) that dimerize after stimulation by ligand and initiate downstream signal. Receptor ligand recognition is redundant to some extent, and affinity varies. Although ERBB2 has none known ligand, information technology becomes reactive after heterodimerization with other ERBB family members, the most preferred and potent combination being with ERBB1, whereas the ERBB3 homodimer remains inactive [57].
The redundancy of this pathway suggests its importance as cells have invested in the mechanisms to make this regulatory pathway fail sound. We own investigated genomic loci for about 60 genes involved in this pathway (Table 2) [56]. Overall, gains were 2.4 times much frequent than losings, and altogether cell lines contained at to the lowest degree three loci of change. Our information revealed that, as expectable, the ERBB2 venue is highly amplified in four cellular telephone lines (UACC-893, ZR-75-30, BT-474 and SK-BR-3), and overexpression has been shown in two of them [9]. Although elaboration of EGFR-interacting genes RECQL5, H3F3B and GRB2 has been described above, other oftentimes altered loci admit c-MYC, LIMK1, PRCKA, CHN2, ERBB2, PYK2, MAP2K3, MAP2K3 and PLG1. Interestingly, T-47D and the 2 ERBB2-overexpressing lines, BT-474 and SK-BR-3, share amplifications at five gene loci: MAP2K6, CHN2, PRKCA, LIMK1 and c-MYC.
Conclusion
We examined the genomes of cardinal commonly used breast cancer cellphone models in unexampled detail for metameric copy list status, cataloging the boundaries of gains and losses throughout these genomes. In gain, we incontestable that copy turn alteration of multiple genic loci involved in the EGF family of pathways is common in these cell lines, which suggests that disruption of this frequently dysregulated pathway in breast cancer may go on at several points in the signal cascade down and that individual disruptions may pass off in combination.
Furthermore, because these cadre lines serve as models for studying the unit biology of breast cancer, it is vital to take into account the potential drop charm of genetical alterations when renderin natural data. For instance, using these lines to study the EGF family of pathways, multiple endogenous genetic alterations may wealthy person a role in biochemical and biological observations. Our work provides a well-rounded lean of high-level divided gains and losses for each genome, providing a database of copy number alterations as a resource for breast Crab research with these cubicle lines.
Abbreviations
- BAC:
-
micro-organism conventionalized chromosome
- CGH:
-
relation genomic hybridization
- CNA:
-
copy number alteration
- EGFR:
-
epidermal ontogeny factor receptor
- Fish:
-
fluorescence in situ hybridization
- kb:
-
kilobases
- MAR:
-
minimum altered region
- Megabyte:
-
megabases
- SMRT:
-
submegabase-resolution tiling solidification
- SNP:
-
lone nucleotide pleomorphism.
References
- 1.
Parkin Decimetre, Bray F, Ferlay J, Pisani P: Ball-shaped Cancer the Crab statistics, 2002. Golden State Cancer the Crab J Clin. 2005, 55: 74-108.
- 2.
Bray F, McCarron P, Parkin DM: The changing global patterns of female breast cancer relative incidence and mortality. Bosom Cancer Res. 2004, 6: 229-239. 10.1186/bcr932.
- 3.
Simpson PT, Reis-Filho JS, Gale T, Lakhani SR: Unit phylogenesis of knocker cancer. J Pathol. 2005, 205: 248-254. 10.1002/way of life.1691.
- 4.
Hanahan D, Weinberg Celestial longitude: The hallmarks of Cancer the Crab. Prison cell. 2000, 100: 57-70. 10.1016/S0092-8674(00)81683-9.
- 5.
Garnis C, Buys TP, Lam WL: Genetic adjustment and gene expression modulation during cancer progression. Mol Cancer. 2004, 3: 9-10.1186/1476-4598-3-9.
- 6.
Kallioniemi OP, Kallioniemi A, Kurisu W, Thor A, Subgenus Chen LC, Smith HS, Waldman Fm, Pinkel D, Gray JW: ERBB2 amplification in breast Cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci U.S.. 1992, 89: 5321-5325.
- 7.
Shimada M, Imura J, Kozaki T, Fujimori T, Asakawa S, Shimizu N, Kawaguchi R: Detection of Her2/neu, c-MYC and ZNF217 gene gain during tit cancer progression using fluorescence in place hybridization. Oncol Rep. 2005, 13: 633-641.
- 8.
Jarvinen TA, Tanner M, Rantanen V, Barlund M, Borg A, Grenman S, Isola J: Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitiveness to topoisomerase 2 inhibitor doxorubicin in titty cancer. Am J Pathol. 2000, 156: 839-847.
- 9.
Lacroix M, Leclercq G: Relevance of front cancer cell lines every bit models for breast tumours: an update. Tit Cancer Res Care for. 2004, 83: 249-289. 10.1023/B:BREA.0000014042.54925.cc.
- 10.
Emens LA, Davidson Atomic number 10: Trastuzumab in breast cancer. Oncology (Williston Park). 2004, 18: 1117-1128.
- 11.
Baselga J: Herceptin alone or in combination with chemotherapy in the treatment of HER2-convinced metastatic breast genus Cancer: pivotal trials. Oncology. 2001, 61 (Suppl 2): 14-21. 10.1159/000055397.
- 12.
Vogel Centiliter, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon Disk-jockey, Murphy M, Novotny WF, Burchmore M, et al: First-seam Herceptin monotherapy in pathological process breast cancer. Oncology. 2001, 61 (Suppl 2): 37-42. 10.1159/000055400.
- 13.
Kallioniemi A, Kallioniemi OP, Piper J, Tanner M, Stokke T, Chen L, Smith HS, Pinkel D, Gray JW, Waldman Atomic number 100: Detection and mapping of amplified Deoxyribonucleic acid sequences in breast cancer by comparative genomic crossing. Proc Natl Acad Sci US Army. 1994, 91: 2156-2160.
- 14.
Kytola S, Rummukainen J, Nordgren A, Karhu R, Farnebo F, Isola J, Larsson C: Body alterations in 15 breast cancer cell lines by comparative genomic hybridization and spectral karyotyping. Genes Chromosomes Genus Cancer. 2000, 28: 308-317. 10.1002/1098-2264(200007)28:3<308::AID-GCC9>3.0.Atomic number 27;2-B.
- 15.
Forozan F, Mahlamaki EH, Monni O, Chen Y, Veldman R, Jiang Y, Gooden Gc, Ethier SP, Kallioniemi A, Kallioniemi OP: Comparative genomic hybridizin analysis of 38 bosom cancer cell lines: a foundation for interpreting cDNA microarray information. Cancer Res. 2000, 60: 4519-4525.
- 16.
Davies JJ, Harriet Wilson IM, Lam WL: Array CGH technologies and their applications to cancer genomes. Chromosome RES. 2005, 13: 237-248. 10.1007/s10577-005-2168-x.
- 17.
Pinkel D, Albertson DG: Array relative genomic cross and its applications in cancer. Nat Genet. 2005, 37 (Suppl): S11-17. 10.1038/ng1569.
- 18.
Collins C, Volik S, Kowbel D, Ginzinger D, Ylstra B, Cloutier T, Hawkins T, Predki P, Martin C, Wernick M, et aluminum: Comprehensive genome sequence analysis of a breast cancer amplicon. Genome Res. 2001, 11: 1034-1042. 10.1101/gr.GR1743R.
- 19.
Albertson DG, Ylstra B, Segraves R, Tom Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D: Quantitative mapping of amplicon social organisation by array CGH identifies CYP24 as a candidate oncogene. Nat Genet. 2000, 25: 144-146. 10.1038/75985.
- 20.
Hodgson JG, Chin K, Collins C, Gray JW: Genome amplification of chromosome 20 in breast cancer. Breast Cancer RES Goody. 2003, 78: 337-345. 10.1023/A:1023085825042.
- 21.
Orsetti B, Nugoli M, Cervera N, Lasorsa L, Chuchana P, Ursule L, Nguyen C, Redon R, du Manoir S, Rodriguez C, et alii: Genomic and expression profiling of chromosome 17 in breast cancer reveals complex patterns of alterations and new candidate genes. Cancer Res. 2004, 64: 6453-6460. 10.1158/0008-5472.CAN-04-0756.
- 22.
Albertson DG: Profiling breast cancer by array CGH. Breast Cancer Res Treat. 2003, 78: 289-298. 10.1023/A:1023025506386.
- 23.
Nessling M, Richter K, Schwaenen C, Roerig P, Wrobel G, Wessendorf S, Fritz B, Bentz M, Sinn HP, Radlwimmer B, et al: Candidate genes in titty cancer revealed by microarray-based relation genomic cross of archived tissue. Cancer Res. 2005, 65: 439-447.
- 24.
Kauraniemi P, Barlund M, Monni O, Kallioniemi A: New amplified and highly expressed genes unconcealed in the ERBB2 amplicon in breast cancer by cDNA microarrays. Cancer Res. 2001, 61: 8235-8240.
- 25.
Monni O, Barlund M, Mousses S, Kononen J, Sauter G, Heiskanen M, Paavola P, Avela K, Chen Y, Bittner ML, et al: Comprehensive copy number and gene expression profiling of the 17q23 amplicon in human breast cancer. Proc Natl Acad Sci America. 2001, 98: 5711-5716. 10.1073/pnas.091582298.
- 26.
Hyman E, Kauraniemi P, Hautaniemi S, Wolf M, Mousses S, Rozenblum E, Ringner M, Sauter G, Monni O, Elkahloun A, et al: Impact of DNA gain along gene verbalism patterns in breast cancer. Cancer Res. 2002, 62: 6240-6245.
- 27.
Charles Joseph Clark J, Edwards S, John M, Flohr P, Gordon T, Maillard K, Giddings I, Brown C, Bagherzadeh A, Campbell C, et al: Identification of amplified and expressed genes in breast Cancer aside comparative hybridisation onto microarrays of arbitrarily selected cDNA clones. Genes Chromosomes Cancer. 2002, 34: 104-114. 10.1002/gcc.10039.
- 28.
Pollack JR, Sorlie T, Perou Atomic number 96, Rees Golden State, Jeffrey SS, Lonning PE, Tibshirani R, Botstein D, Borresen-Dale AL, Brownish PO: Microarray analysis reveals a major direct use of Desoxyribonucleic acid simulate number alteration in the transcriptional program of human tit tumors. Proc Natl Acad Sci Army. 2002, 99: 12963-12968. 10.1073/pnas.162471999.
- 29.
Ishkanian AS, Malloff CA, Watson SK, DeLeeuw RJ, Chi B, Coe BP, Snijders A, Albertson DG, Pinkel D, Marra MA, et al: A tiling resolution DNA microarray with complete coverage of the human genome. Nat Genet. 2004, 36: 299-303. 10.1038/ng1307.
- 30.
Delaware Leeuw RJ, Davies JJ, Rosenwald A, Bebb G, Gascoyne RD, Dyer MJ, Staudt LM, Martinez-Climent JA, Lam WL: Comprehensive whole genome array CGH profiling of mantle cell lymphoma model genomes. Hum Mol Genet. 2004, 13: 1827-1837. 10.1093/hmg/ddh195.
- 31.
Watson SK, deLeeuw RJ, Ishkanian AS, Malloff CA, Lam WL: Methods for high throughput validation of amplified fragment pools of BAC Deoxyribonucleic acid for constructing high resolution CGH arrays. BMC Genomics. 2004, 5: 6-10.1186/1471-2164-5-6.
- 32.
Chi B, DeLeeuw RJ, Coe BP, MacAulay C, Lam WL: SeeGH – a software puppet for visualization of whole genome range comparative genomic hybridization data. BMC Bioinformatics. 2004, 5: 13-10.1186/1471-2105-5-13.
- 33.
ArrayCGH. [http://www.arraycgh.Ca]
- 34.
Jong K, Marchiori E, Meijer G, Vaart AV, Ylstra B: Breakpoint identification and smoothing of align relative genomic hybridizin data. Bioinformatics. 2004, 20: 3636-3637.
- 35.
VU Micro-Range Information Analysis. [http://www.a couple of.vu.nl/~vumarray/]
- 36.
Henderson LJ, Lestou VS, Ludkovski O, Robichaud M, Chhanabhai M, Gascoyne RD, Klasa RJ, Connors JM, Marra MA, Horsman DE, et alibi: Delineation of a token region of deletion at 6q16.3 in follicular lymphoma and construction of a bacterial artificial chromosome contig spanning a 6-megabase domain of 6q16-q21. Genes Chromosomes Cancer the Crab. 2004, 40: 60-65. 10.1002/gcc.20013.
- 37.
Savinainen KJ, Linja MJ, Saramaki OR, Tammela TL, Chang GT, Brinkmann AO, Visakorpi T: Expression and copy number analysis of TRPS1, EIF3S3 and MYC genes in breast and prostate cancer. Br J Cancer the Crab. 2004, 90: 1041-1046. 10.1038/sj.bjc.6601648.
- 38.
Rummukainen J, Kytola S, Karhu R, Farnebo F, Larsson C, Isola JJ: Aberrations of chromosome 8 in 16 breast cancer cell lines past relative genomic hybridization, fluorescence in situ crossing, and spectral karyotyping. Cancer Genet Cytogenet. 2001, 126: 1-7. 10.1016/S0165-4608(00)00387-3.
- 39.
Zhao X, Li C, Paez JG, Chin K, Janne PA, Chen Atomic number 90, Girard L, Minna J, Christiani D, Leo C, et al: An integrated view of copy number and allelic alterations in the Cancer the Crab genome using single nucleotide pleomorphism arrays. Cancer RES. 2004, 64: 3060-3071. 10.1158/0008-5472.Derriere-03-3308.
- 40.
Maass N, Rosel F, Schem C, Hitomi J, Jonat W, Nagasaki K: Gain of the BCAS2 cistron at chromosome 1p13.3-21 in human primary front Cancer. Cancer Lett. 2002, 185: 219-223. 10.1016/S0304-3835(02)00286-0.
- 41.
Qi C, Zhu YT, Chang J, Yeldandi AV, Rao MS, Zhu YJ: Potentiation of estrogen receptor transcriptional activity by white meat cancer amplified sequence 2. Biochem Biophys Res Commun. 2005, 328: 393-398. 10.1016/j.bbrc.2004.12.187.
- 42.
Nagasaki K, Maass N, Manabe T, Hanzawa H, Tsukada T, Kikuchi K, Yamaguchi K: Identification of a novel cistron, DAM1, amplified at chromosome 1p13.3-21 region in human breast cancer cadre lines. Cancer Lett. 1999, 140 (1–2): 219-226. 10.1016/S0304-3835(99)00091-9.
- 43.
Lapuk A, Volik S, Vincent R, Chin K, Kuo WL, Diamond State Erica Jong P, Collins C, Gray JW: Computational BAC clone contig assembly for comprehensive genome analysis. Genes Chromosomes Cancer. 2004, 40: 66-71. 10.1002/gcc.20016.
- 44.
Falck J, Coates J, Stonewall Jackson SP: Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA impairment. Nature. 2005, 434: 605-611. 10.1038/nature03442.
- 45.
Jaquemar D, Schenker T, Trueb B: An ankyrin-comparable protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999, 274: 7325-7333. 10.1074/jbc.274.11.7325.
- 46.
Dantzig AH, Hoskins JA, Tabas LB, Glary S, Shepard RL, Jenkins IL, Duckworth District of Columbia, Sportsman Junior, Mackensen D, Rosteck PR, et al: Association of intestinal peptide transmit with a protein related the cadherin superfamily. Scientific discipline. 1994, 264: 430-433.
- 47.
Mom Y, Pannicke U, Schwarz K, Lieber MR: Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end connection and V(D)J recombination. Cadre. 2002, 108: 781-794. 10.1016/S0092-8674(02)00671-2.
- 48.
Fletcher Gc, Patel S, Tyson K, Adam PJ, Schenker M, Longshoreman JA, Daviet L, Legrain P, Parekh R, Harris Aluminium, et al: hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with estrogen sense organ-prescribed breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Malignant neoplastic disease. 2003, 88: 579-585. 10.1038/sj.bjc.6600740.
- 49.
Snijders AM, Nowak N, Segraves R, Blackwood S, Chromatic N, Conroy J, Hamilton G, Hindle Alaska, Huey B, Kimura K, et al: Assembly of microarrays for genome-panoptic measurement of DNA copy numeral. Nat Genet. 2001, 29: 263-264. 10.1038/ng754.
- 50.
Xie D, Jauch A, Henry Valentine Miller CW, Bartram Atomic number 24, Koeffler HP: Discovery of over-expressed genes and hereditary alterations in bosom cancer cells exploitation a combination of crushing subtractive hybridization, complex FISH and relative genomic hybridization. Int J Oncol. 2002, 21: 499-507.
- 51.
Watson MB, Bahia H, Ashman JN, Berrieman HK, Drew P, Lind MJ, Greenman J, Cawkwell L: Chromosomal alterations in breast cancer revealed by multi-colored fluorescence in place hybridization. Int J Oncol. 2004, 25: 277-283.
- 52.
Barlund M, Tirkkonen M, Forozan F, Tanner MM, Kallioniemi O, Kallioniemi A: Increased copy number at 17q22-q24 away CGH in white meat cancer is payable to high-even elaboration of two separate regions. Genes Chromosomes Cancer. 1997, 20: 372-376. 10.1002/(SICI)1098-2264(199712)20:4<372::AID-GCC8>3.0.CO;2-Z.
- 53.
Lowenstein EJ, Daly RJ, Batzer AG, Cardinal W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J: The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cellular telephone. 1992, 70: 431-442. 10.1016/0092-8674(92)90167-B.
- 54.
Wu GJ, Sir Clive Marles Sinclair CS, Paape J, Ingle JN, Roche PC, James I CD, Couch FJ: 17q23 amplifications in breast genus Cancer involve the PAT1, RAD51C, PS6K, and SIGma1B genes. Cancer Res. 2000, 60: 5371-5375.
- 55.
Bhargava R, Gerald WL, Li AR, Pan Q, Lal P, Ladanyi M, Chen B: EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein verbalism and HER-2 position and absence of EGFR-activating mutations. Mod Pathol. 2005, 18: 1027-1033. 10.1038/modpathol.3800438.
- 56.
Oda K, Matsuoka Y, Funahashi A, Kitano H: A comprehensive pathway represent of epidermal increase factor receptor signaling. Mol Syst Biol. 2005, 1: msb4100014-E1-msb4100014-E17. 10.1038/msb4100014.
- 57.
Graus-Porta D, Beerli RR, Daly JM, Hynes NE: ErbB-2, the preferent heterodimerization mate of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16: 1647-1655. 10.1093/emboj/16.7.1647.
Acknowledgements
We thank Chadic Malloff and Jonathan Davies for their guidance in information analytic thinking and manuscript preparation, Sean Minaker and Mother Theresa Mastracci for providing interphase slides, and Carol Cheng for her subject area assistance. This work was supported by monetary resource from Genome Canada/Genome British Columbia.
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they give birth no competing interests.
Authors' contributions
As performed the raiment CGH experiments, data analysis and drafted the manuscript. WLL is the Principal Investigator. Both authors participated in the development of concepts and frame for the manuscript, the generation of figures, multiple rounds of text editing, and fact checking. Some authors read and approved the final holograph.
Electronic supplementary material
Authors' original submitted files for images
Rights and permissions
About this article
Cite this clause
Shadeo, A., Lam, W.L. Broad copy number profiles of knocker cancer cellular telephone model genomes. Breast Cancer Res 8, R9 (2006). https://Department of the Interior.org/10.1186/bcr1370
-
Received:
-
Revised:
-
Accepted:
-
Promulgated:
-
Interior Department : https://DoI.org/10.1186/bcr1370
Keywords
- Bacterial Artificial Chromosome
- Microorganism Factitious Chromosome Dead ringer
- Array Comparative Genomic Hybridization
- Copy Number Alteration
- Short-lived Receptor Potential Cation Duct
Where to Download Genotype Information for Mcf7 Cell Lines
Source: https://breast-cancer-research.biomedcentral.com/articles/10.1186/bcr1370
0 Response to "Where to Download Genotype Information for Mcf7 Cell Lines"
Post a Comment